Malignant tumor is one of the most dangerous fatal diseases to human being. Molecular targeting anticancer drugs have attracted an increasing attention for their high selectivity and low toxicity. The researchers in Wang’s Group of the CAS Key Laboratory of Analytical Chemistry for Living Biosystems have designed and synthesized a series of organic protein kinase inhibitors which contain potential sites for metal coordination (Eur. J. Med. Chem. 2013, 61, 84–94; Bioorg. Med. Chem. Lett. 2011, 21, 6964–6968), and some of which have been characterized to be excellent EGFR (Epidermal growth factor receptor) inhibitors by a novel MS-based quantitative method (Anal. Chem. 2012, 84, 2284-2291).
Based on previous study and the principle of single-molecule-multi-targeting for drug design, they designed and synthesized a series of Ru-based complexes with the EGFR inhibitors they developed as a ligand. The in vitro screening revealed that the as-prepared ruthenium complexes are highly active to inhibit enzymatic activity of EGFR. More interestingly, one of the organometallic ruthenium complex exhibits higher activity to induce apoptosis than the molecular targeting anticancer drug gefitinib which has been widely used for the clinic treatment of advanced non-small cell lung cancer. This work is a leading contribution in developing novel metal-based multi-targeting anticancer drugs, and has been recently published online in Chemical Communications (2013, DOI: 10.1039/c3cc43000f ).
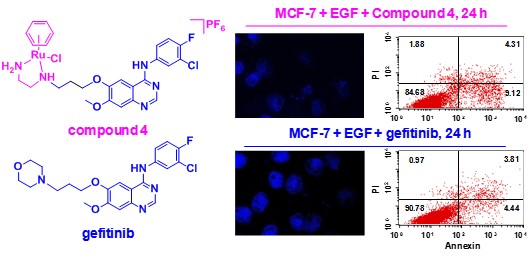
Figure 1 Chemical structures of a synthesized ruthenium arene complex (top left) and the molecular targeting anticancer drug gefitinib (bottom left) and the comparison of ability of these two compounds to induce apoptosis of MCF-7 human breast cancer cells (right).
